Abstract
Background: In CMML, allogeneic stem cell transplantation (alloSCT) remains the only potentially curative treatment modality. However, despite the advent of reduced intensity conditioning (RIC) and advances in supportive care, transplant results in CMML are still hampered by relatively high risks of non-relapse mortality (NRM) and relapse, and novel transplant strategies aimed at reducing NRM while preserving the anti-leukemic activity are needed. Prompted by the results obtained in patients with acute myeloid leukemia considered ineligible for conventional conditioning that demonstrated low 2-year NRM rates and satisfactory disease control, we adopted fractionated intermediate-dose total body irradiation (TBI) in combination with fludarabine as routine conditioning for CMML from 2012 onwards. This retrospective analysis investigated the outcomes of patients with CMML undergoing alloSCT focusing on the impact of the conditioning treatment.
Patients and Methods: Patients were included if they had been allografted for CMML at our institution and had written informed consent to data collection.
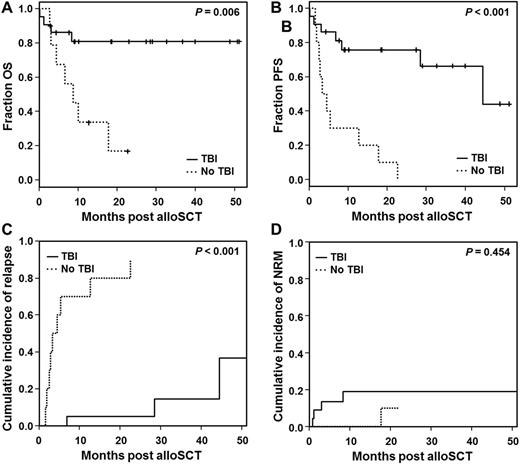
Results: A total of 29 patients (median age 59 years) undergoing alloSCT between 2002 and 2016 in our institution met the eligibility criteria. Three patients underwent second alloSCT after CMML relapse, therefore a total of 32 transplants are reported. Diagnoses were CMML-1 in 10 (31%), CMML-2 in 8 (25%) and secondary AML in 14 cases (44%). All patients with secondary AML had confirmed antecedent CMML prior to transformation. The risk categories according to HCT-specific CPSS (i.e. CPSS prior to alloSCT) were low/intermediate-1 in 11 (61%), intermediate-2/high in 7 (39%) cases and not applicable for patients with secondary AML. Treatment prior to alloSCT was hypomethylating agents (HMA) and AML-like chemotherapy in 10 (31%) and 12 (38%) cases, respectively. Seven patients were previously untreated. Ten patients received chemotherapy-based RIC, whereas in 22 (69%) cases including all 3 secondary transplants the conditioning regimen consisted of fractionated 6 or 8 Gray TBI in combination with fludarabine. Disease and transplant characteristics including disease stage, bone marrow blast count, karyotype, pre-treatment and comorbidities did not differ between the TBI (n=22) and TBI-free (n=10) cohort except for year of transplant, with the majority of patients in the TBI-free cohort being transplanted prior to 2013 (P=0.024). In univariable analysis with overall survival (OS) and progression-free survival (PFS) as endpoints, TBI-based conditioning was associated with a significantly improved OS (HR 0.21, P=0.013) and PFS (HR 0.14, P <0.001). Furthermore, previous treatment with AML-like chemotherapy and transplantation from related donor were associated with a trend towards inferior OS but not PFS. Consequently, for patients who underwent allografting after TBI-containing conditioning, estimated probabilities of OS and PFS at 1 year post-transplant was 81% (95%CI 57-92) and 76% (95%CI 51-89), respectively, which was substantially higher as compared to patients who received TBI-free conditioning (OS 34% 95%CI 8-63 and PFS 30% 95%CI 7-58 at 1 year) (Figure 1A and B). Improved survival was due to substantially lower relapse incidence in the TBI-based (cumulative incidence at 1 year: 5% 95%CI 0-21) as compared to the TBI-free cohort (70% 95%CI 28-90) (Figure 1C). In contrast, cumulative incidence of NRM did not differ between the cohorts (P=0.454) (Figure 1D). In multivariable analyses type of conditioning, pre-treatment and donor type as covariates, TBI-based conditioning was significantly associated with prolonged PFS (HR 0.10, P <0.001).
Conclusion: This data suggest that alloSCT based on intermediate-dose TBI conditioning might be associated with improved outcome in patients with advanced CMML.
Hegenbart: Janssen: Honoraria, Speakers Bureau; Prothena: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Dreger: medac: Other: Travel grants; Riemser: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; medac: Other; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; medac: Other: Travel grants; medac: Other: Travel grants; medac: Other; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; medac: Other; Gilead: Consultancy, Speakers Bureau; medac: Other; medac: Other: Travel grants; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau; medac: Other: Travel grants; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Jansen: Consultancy; Riemser: Consultancy, Research Funding; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; AbbVie: Consultancy, Other: Travel grants, Speakers Bureau; Jansen: Consultancy; Jansen: Consultancy; Gilead: Consultancy, Speakers Bureau; Gilead: Consultancy, Speakers Bureau. Luft: Neovii: Research Funding; Alexion: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal